Anti-oxidant effect of Flemingia stricta Roxb. leaves methanolic extract
Abstract
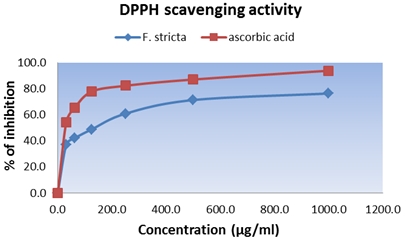
Aim of the study was to evaluate the possible anti-oxidant activity of Flemingia stricta leaf extract. In antioxidant study, plant crude methanol extract was evaluated for 1,1-diphenyl,2-picrylhydrazyl (DPPH) and reducing power capacity. Moreover, total phenolic and total flavonoid content of plant extracts were determined and expressed in mg of gallic acid equivalent per gram of dry sample (mg GAE/g dry weight). In the DPPH free radical scavenging assay, methanol extract showed concentration dependent inhibition of the free radicals. IC50 of ascorbic acid and F. stricta leaves were 4.25 µg/ml and 320.47 µg/ml respectively. In case of reducing capacity, the methanol extract at concentrations of 25, 50, 100, 200, 400 µg/ml, the absorbances were 0.56, 0.92, 1.41, 1.76, 2.23, respectively. Total phenolic content was estimated by gallic acid and expressed as milligrams of gallic acid equivalent (GAE). The methanol extracts contained a considerable amount of phenolic contents of 482±8.72 of GAE/g of extract and the total flavonoid content of the F. stricta leaf was estimated by using aluminium chloride colorimetric technique and found that the extract contained flavonoid content 340.625±4.50 of GAE/g of extract. These results suggested that the methanol extract of F. stricta Roxb. possess anti-oxidant activity.
Downloads
References
2. http://www.legumes-online.net/ildis/aweb/td076/td_16024.htm [Accessed on 2018 Aug 5].
3. Rahman MA, Uddin SB, Wilcock CC. Medicinal plants used by chakma tribe in hill tracts districts of Bangladesh. Indian J Tradit Knowl. 2007; 6(3): 508-517.
4. Uddin SN. Traditional uses of ethnomedicinal plants of the chittagong hill tracts. Bangladesh National Herbarium, Bangladesh, 2006: 992.
5. Khisha T, Karim R, Chowdhury SR, Banoo R. Ethnomedical studies of chakma communities of chittagong hill tracts. Bangladesh. Bangl Pharm J. 2012; 15(1): 59-67.
6. Agati G, Azzarello E, Pollastri S, Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 2012; 196: 67-76.
7. Ramel F, Mialoundama AS, Havaux M. Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J Exp Bot. 2013; 3: 799-805.
8. Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010; 15: 13-52.
9. Alam MN, Hossain MM, Rahman MM, Subhan N, Mamun MAA, Ulla A, et al. Astaxanthin prevented oxidative stress in heart and kidneys of isoproterenol-administered aged rats. J Diet Suppl. 2018; 15(1): 42-54.
10. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002; 82: 47-95.
11. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007; 39: 44-84.
12. Cook NC, Samman S. Flavonoids-chemistry, metabolism, cardio protective effects, and dietary sources. Nut Biochem. 1996; 7: 66-76.
13. Kumpulainen JT, Salonen JT. Natural antioxidants and anticarcinogens in nutrition, health and disease. The Royal Society of Chemistry, UK. 1990: 178-187.
14. Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998; 78: 547-581.
15. Trease G, Evans SM. Pharmacognosy. 15th edn. London: Bailer Tindal; 2002: 23-67.
16. Harborne JB. Phytochemical methods - a guide to modern techniques of plant analysis. 2nd edn. London: Chapman and Hall; 1984: 4-16.
17. Ghani, A. Medicinal plants of Bangladesh: chemical constituents and uses. Asiatic Society of Bangladesh, Dhaka, 1998.
18. Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Scient World J. 2017, ID 5873648.
19. Braca A, Tommasi ND, Bari LD, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia terapotensis. J Nat Prod. 2001; 64(7): 892-895.
20. Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jap J Nutr.1986; 44: 307-315.
21. Harborne JB. Phytochemical methods. London: Chapman and Hall, 1973: 49-188.
22. Wang SY, Jiao H. Correlation of antioxidant capacities to oxygen radical scavenging enzyme activities in blackberry. J Agric Food Chem. 2000; 48(11): 5672-5676.
23. Bashir S, Gilani A. Studies on the antioxidant and analgesic activities of Aztec marigold (Tagetes erecta) flowers. Phytother Res. 2008; 22(12): 1692-1694.
24. Soare J, Dinis T, Cunha A, Almeida L. Antioxidant activities of some extracts of Thymus zygis. Free Radic Res. 1997; 26(5): 469-478.
25. Nunes PX, Silva SF, Guedes RJ, Almeida S. Biological oxidations and antioxidant activity of natural products, phytochemicals as nutraceuticals. Global approaches to their role in nutrition and health, 2012.
26. Krishnaiah D, Sarbatly R, Nithyanandam RR. A review of the antioxidant potential of medicinal plant species. Food Bioprod Process. 2011; 89: 217-233.
27. Gordon MH. The mechanism of antioxidant action in vitro. In: Food antioxidants. Hudson BJ, ed. London: Elsevier Applied Science, 1990: 1-18.
28. Shahidi F, Wanasundara P. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992; 32: 67-103.
29. Hagerman A, Riedl K, Jones G, Sovik K, Ritchard N, Hartzfeld P. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998; 46: 1887-1892.
30. Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999; 65: 337-353.
31. Montoro P, Braca A, Pizza C, De Tommasi N. Structure-antioxidant activity relationships of flavonoids isolated from different plant species. Food Chem. 2005; 92: 349-355.
32. Bravo L. Polyphenols: chemistry, dietary sources, metabolism and nutritional significance. Nutr Rev. 1998; 56: 317-333.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




