Dynamics of oxygen consumption during the formation of the anoxic zone in aquatic environment
Abstract
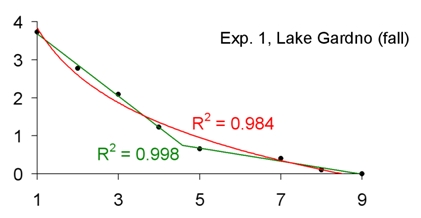
Anoxic environments and communities of anaerobic organisms are encountered in aquatic environments and biotechnological reactors. Because of their importance, they are continuously studied. In this study, the dynamics of oxygen removal were observed during experiments reproducing the formation of the anoxic zone. Seven experiments were performed in an aquarium (volume: 60 l) with bottom sediments and water collected from different aquatic environments (river, pond, eutrophic lake, sea). To exclude reaeration, the water was isolated from the air by a layer of liquid paraffin. Below the paraffin layer the water was periodically mixed with a stirrer and sampled for oxygen concentration. Initially, a high rate of oxygen consumption was observed. Later, at low oxygen concentrations, the oxygen removal rate switched to a much lower one. Anoxic conditions were observed after 4-20 days of incubation, depending on the experiment. The point at which the microbial community converted from aerobic respiration to anaerobic metabolism was distinct and was observed at an oxygen concentration of 0.26-1.41 mg/l, depending on the experiment. The experiments were accompanied by bacterial counts and analyses of ciliate communities. The study indicates how the disappearance of oxygen during anoxic zone formation should be modeled, and provides data on the oxygen removal rates associated with aerobic and anaerobic communities of microorganisms.
Downloads
References
2. Powilleit M, Kube J. Effects of severe oxygen depletion on macrobenthos in the Pomeranian Bay (southern Baltic Sea): a case study in a shallow, sublittoral habitat characterised by low species richness. J Sea Res. 1999; 42: 221-234.
3. Rasmussen B, Gustafsson BG, Ærtebjerg G, Lundsgaard C. Oxygen concentration and consumption at the entrance to the Baltic Sea from 1975 to 2000. J Marine Syst. 2003; 42: 13-30.
4. Liu L, Lwiza KMM, Taylor GT. Importance of the bacterial dynamics in model simulations of seasonal hypoxia. Cont Shelf Res. 2015; 105: 1-17.
5. González Siso MI, Becerra M, Lamas Maceiras M, Vizoso Vázquez A, Cerdán ME. The yeast hypoxic responses, resources for new biotechnological opportunities. Biotechnol Lett. 2012; 34: 2161-2173.
6. Martínez-García CG, Fall C, Olguín MT. Activated sludge mass reduction and biodegradability of the endogenous residues by digestion under different aerobic to anaerobic conditions: comparison and modeling. Biores Technol. 2016; 203: 32-41.
7. Fenchel T, Kristensen LD, Rasmussen L. Water column anoxia: vertical zonation of planktonic protozoa. Mar Ecol Prog Ser. 1990; 62: 1-10.
8. Fenchel T, Finlay BJ. Ecology and evolution in anoxic worlds. Oxford, New York, Tokyo, Oxford University Press, 1995.
9. Rosenberg R, Nilsson HC, Diaz RJ. Response of benthic fauna and changing sediment redox profiles over a hypoxic gradient. Est Coast Shelf Sci. 2001; 53: 343-350.
10. Fenchel T, Finlay BJ. Oxygen toxicity, respiration and behavioural responses to oxygen in free-living anaerobic ciliates. J Gen Microbiol. 1990; 136: 1953-1959.
11. Rocke E, Liu H. Respiration, growth and grazing rates of three ciliate species in hypoxic conditions. Mar Pollut Bull. 2014; 85: 410-417.
12. DeRisi JL, Iyer VR, Brown PO. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997; 278: 680-686.
13. Kawasaki S, Mimura T, Satoh T, Takeda K, Niimura Y. Response of the microaerophilic Bifidobacterium species, B. boum and B. thermophilum, to oxygen. Appl Environ Microbiol. 2006; 72: 6854-6858.
14. Livingstone DM, Imboden DM. The prediction of hypolimnetic oxygen profiles: a plea for a deductive approach. Can J Fish Aquat Sci. 1996; 53: 924-932.
15. Rychert K. Communities of heterotrophic protists (protozoa) in the near-bottom zone of the Gdańsk Basin. Oceanol Hydrobiol Stud. 2011; 40: 68-73.
16. Dean WE Jr. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: comparison with other methods. J Sediment Petrol. 1974; 44: 242-248.
17. Jørgensen BB, Cohen Y, Revsbech NP. Transition from anoxygenic to oxygenic photosynthesis in a Microcoleus chthonoplastes cyanobacterial mat. Appl Environ Microbiol. 1986; 51(2): 408-417.
18. Kirchmann H, Ewnetu W. Biodegradation of petroleum-based oil wastes through composting. Biodegradation. 1998; 9: 151-156.
19. Hobbie JE, Daley RJ, Jasper S. Use of nucleopore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977; 33: 1225-1228.
20. Utermöhl H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt Int Ver Limnol. 1958; 9: 1-38.
21. Foissner W, Berger H. A user-friendly guide to the ciliates (Protozoa, Ciliophora) commonly used by hydrobiologists as bioindicators in rivers, lakes, and waste waters, with notes on their ecology. Freshwater Biol. 1996; 35: 375-482.
22. Dolan JR, Coats DW. Changes in fine-scale vertical distributions of ciliate microzooplankton related to anoxia in Chesapeake Bay waters. Mar Microb Food Webs. 1991; 5: 81-93.
23. Codispoti LA, Yoshinari T, Devol AH. Suboxic respiration in the oceanic water column. In: del Giorgio PA, Williams PjleB, eds. Respiration in aquatic ecosystems. New York, Oxford University Press, 2005: 225-247.
24. Lu H, Kalyuzhnaya M, Chandran K. Comparative proteomic analysis reveals insights into anoxic growth of Methyloversatilis universalis FAM5 on methanol and ethanol. Environ Microbiol. 2012; 14: 2935-2945.
25. Weber F, Anderson R, Foissner W, Mylnikov AP, Jürgens K. Morphological and molecular approaches reveal highly stratified protist communities along Baltic Sea pelagic redox gradients. Aquat Microb Ecol. 2014; 73: 1-16.
26. Vaqué D, Casamayor EO, Gasol JM. Dynamics of whole community bacterial production and grazing losses in seawater incubations as related to the changes in the proportions of bacteria with different DNA content. Aquat Microb Ecol. 2001; 25: 163-177.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




