Hyoscyamus muticus L. subsp. falezlez methanolic extract: phytochemical composition and biological activities
Abstract
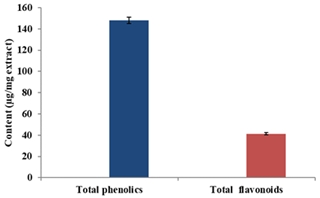
This study aims to assess the phytochemical analysis and evaluate the antioxidant and antimicrobial activities of the methanolic extract obtained from the Algerian Hyoscyamus muticus L. subsp. falezlez leaves of Timimoun region. Methanolic extract of the plant contained the highest quantity of phenolics (148.00 ± 3.07 µg GAE/mg extract) and flavonoids (41.43 ± 0.90 µg QE/mg extract). The High-Performance Liquid Chromatography (HPLC) results showed dominance in the phenolic compounds: orientin, vitexin 2-O-rhamnoside and n-OH-cinnamic acid. Eight metabolites were identified and quantified by Gas Chromatography-Mass Spectrometry (GC-MS) which included five fatty acids, one dicarboxylic acid derivative, one bicyclic hydrocarbon and one fatty acid derivate. The GC-MS analysis revealed that palmitic acid (32.56%), linolenic acid (21.34%) and linoleic acid (11.24%) were the three major components. The methanolic extract showed an antioxidant activity for DPPH, ABTS, reducing power and phenanthroline assays. The strongest antioxidant activity was obtained with phenanthroline assay (value of A0.5 <3.125 µg/mL). The antimicrobial investigation on thirteen microbial strains revealed that the methanolic extract showed low to moderate antibacterial activity against the Gram-positive and negative tested bacteria and no antifungal activity on all the tested fungi. This work suggests the use of leaves from H. muticus L. subsp. falezlez as a source of bioactive compounds with applications in the pharmaceutical, cosmetic and food industries.
Downloads
References
2. Delgoda R, Murray J. Evolutionary perspectives on the role of plant secondary metabolites. Pharmacogn. 2017: 93-100.
3. Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. 2018; 5(93): 1-16.
4. Ramadan FM, Zayed R, El-Shamy H. Screening of bioactive lipids and radical scavenging potential of some Solanaceae plants. Food Chem. 2007; 103(3): 885-890.
5. Gulcin İ. Antioxidants and antioxidant methods: an updated overview. Arch Toxicol. 2020; 94(3): 651-715.
6. Lim Y, Murtijaya J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT. 2007; 40(9): 1664-1669.
7. Yu M, Gouvinhas I, Rocha J, Barros AI. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci Rep. 2021; 11(1): 10041.
8. Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathogens Global Health. 2015; 109(7): 309-318.
9. Tabassam Q, Mehmood T, Ahmed S, Saeed S, Raza AR, Anwar F. GC-MS metabolomics profiling and HR-APCI-MS characterization of potential anticancer compounds and antimicrobial activities of extracts from Picrorhiza kurroa. J Appl Biomed. 2021; 19(1): 26-39.
10. Quezel P, Santa S. Nouvelle flore de l’Algérie et des régions désertiques méridionales. Tome II, édition Centre National de la Recherche Scientifique, Paris, France. 1963.
11. Simpson MG. Plant systematic. Academic Press: Cambridge, MA, USA. 2019.
12. Almalki M. In vitro antibacterial, antifungal and other medical properties of endangered medicinal plant seeds. Pharmacol Pharma. 2017; 8: 189-204.
13. Al-Snafi AE. Therapeutic importance of Hyoscyamus species grown in Iraq (Hyoscyamus albus, Hyoscyamus niger and Hyoscyamus reticulates) - A review. IOSR J Pharm. 2018; 8(6): 18-32.
14. Al-Tohamy R, Ali SS, Saad-Allah K, Fareed M, Ali A, El-Badry A, et al. Phytochemical analysis and assessment of antioxidant and antimicrobial activities of some medicinal plant species from Egyptian flora. J Appl Biomed. 2018; 16(4): 289-300.
15. Elsharkawy ER, Ed-dra A, Abdallah EM, Ali AMH. Antioxidant, antimicrobial and antifeedant activity of phenolic compounds accumulated in Hyoscyamus muticus L. Afr J Biotechnol. 2018; 17(10): 311-321.
16. Guler GO. Studies on antioxidant properties of the different solvent extracts and fatty acid composition of Hyoscyamus reticulatus L. J Food Biochem. 2012; 36(5): 532-538.
17. Mohammad MK, Almasri IM, Tawaha K, Issa A, Al-Nadaf A, Hudaib M, et al. Antioxidant, antihyperuricemic and xanthine oxidase inhibitory activities of Hyoscyamus reticulatus. Pharm Biol. 2010; 48(12): 1376-1383.
18. Sahki A, Boutamine-Sahki R. Le Hoggar promenade botanique. Edition Esope. Lion, France. 2004.
19. Ayari-Guentri S, Djemouai N, Gaceb-Terrak R, Rahmania F. Chemical composition and antioxidant activity of Hyoscyamus muticus L. subsp. falezlez (Coss.) Maire from Algeria. J Essent Oil-Bear Plants. 2017; 5(20): 1370-1379.
20. Ramdane F, Hadj Mahammed M, Didi Ould Hadj M, Chanai A, Hammoudi R, Hillali N, et al. Ethnobotanical study of some medicinal plants from Hoggar, Algeria. J Med Plant Res. 2015; 9(30): 820-827.
21. Alizadeh A, Moshiri M, Alizadeh J, Balali-Mood M. Black henbane and its toxicity - a descriptive review. Avicenna J Phytomed. 2014; 4(5):297-311.
22. Ould El Hadj MD, Hadj-Mahammed M, Zabeirou H. Place des plantes spontanées dans la médicine traditionnelle de la région de Ouargla (Sahara septentrional Est). Courr Sav. 2003; 3: 47-51.
23. Kebaili Z, Hameurlaine S, Fellah O, Djermane M, Gherraf N, Zellagui A, et al. Assessment of alkaloid content and antibacterial activity of Hyoscyamus albus and Hyoscyamus muticus collected in two different climatic regions in Algeria. J Biochem Tech. 2019; 10(1): 1-6.
24. Nora L, Dalmazo GO, Nora FR, Martin C. Successful regeneration of fertile stably transformed tropane alkaloid-producing plant (Hyoscyamus muticus L.) with PVX-Gus-(astr1 or astr2)-nptii constructs. PCTOC. 2021; 145(3): 517-531.
25. Journal officiel de la république algérienne (JORA). 2012; 12(3): 12-33.
26. Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth Enzymol. 1999; 299: 152-178.
27. Moreno MIV, Isla MI, Sampietro AR, Vattuone MA. Comparison of the free radical scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 2000; 71: 109-114.
28. Goren AC, Kilic T, Dirmenci T, Bilsel G. Chemotaxonomic evaluation of Turkish species of Salvia: fatty acid composition of seed oil. Biochem Sys Ecol. 2006; 34: 160-164.
29. Adams RP. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Allured publishing corporation, Illinois, USA. 2001.
30. Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958; 4617(181): 1119-1200.
31. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999; 26: 1231-1237.
32. Oyaizu M. Studies on products of browning reactions: antioxidative activities of browning reaction prepared from glucosamine. Jap J Nutr Diet. 1986; 44: 307-315.
33. Szydlowska-Czerniaka A, Dianoczki C, Recseg K, Karlovits G, Szlyk E. Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophotometric methods. Talanta. 2008; 76 (4): 899-905.
34. Hayes AJ, Markovic B. Toxicity of Australian essential oil Backhousia citriodora Part 1 antimicrobial activity and in vitro cytotoxicity. Food Chem Toxicol. 2002; 4: 949-964.
35. Wade D, Silveira A, Rollins Smith L, Bergman T, Silberring J, Lankinen H. Hematological and antifungal properties of temporin A and acecropin A-temporin A hybrid. Acta Biochim Pol. 2001; 48: 1185-1189.
36. Kalia K, Sharma K, Singh HP, Singh B. Effects of extraction methods on phenolic contents and antioxidant activity in aerial parts of Potentilla atrosanguinea Lodd. and quantification of its phenolic constituents by RP-HPLC. Agric J Food Chem. 2008; 56(21): 10129-10134.
37. Mohd I, Nudrat F. Phytochemical and antimicrobial screening of Hyoscyamus muticus, a plant found in the northern border region of Saudi Arabia. Indo Am J P Sci. 2017; 4(5): 1216-1220.
38. Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004; 74: 2157-2184.
39. Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Eslami B, Ehsanifar S. Antioxidant activity of Hyoscyamus squarrosus fruits. Pharmacol online. 2009; 2: 644-650.
40. Jassbi A, Miri R, Masroorbabanari M, Asadollahi M, Attarroshan M, Baldwin IT. HPLC DAD-ESIMS analyses of Hyoscyamus niger and H. reticulatus for their antioxidant constituents. Austin Chromatogr. 2014; 1(5): 1022.
41. Gill NS, Arora R, Kumar SR. Evaluation of antioxidant, anti-inflammatory and analgesic potential of the Luffa acutangula Roxb. varamara. Res J Phytochem. 2011; 5: 201-208.
42. Nayak D, Ashe S, Rauta PR, Bismita Nayak B. Assessment of antioxidant, antimicrobial and anti-osteosarcoma potential of four traditionally used Indian medicinal plants. J Appl Biomed. 2017; 15: 119-132.
43. Verma SK, Singh SK, Mathur A, Singh S. In vitro cytotoxicity of Argemone mexicana against different human cancer celllines. Int J Chem Env Pharm Res. 2010; 1(1): 37-39.
44. Lam KY, Ling AP, Koh RY, Wong YP, Say YH. A review on medicinal properties of Orientin. Adv Pharmacol Sci. 2016; 2016: 1-9.
45. Lee W, Bae JS. Antithrombotic and antiplatelet activities of orientin in vitro and in vivo. J Funct Foods. 2015; 17: 388-398.
46. Praveena R, Sadasivam K, Deepha V, Sivakumar R. Antioxidant potential of orientin: a combined experimental and DFT approach. J Mol Struct. 2014; 1061: 114-123.
47. He M, Min JW, Kong WL, He XH, Li JX, Peng BW. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia. 2016; 115: 74-85.
48. Ruwizhi N, Aderibigbe BA. Cinnamic acid derivatives and their biological efficacy. Int J Mol Sci. 2020; 21(16): 5712.
49. Sova M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev Med Chem. 2012; 12(8): 749-767.
50. Sharma P, Vijayvergia R. In vitro α-amylase inhibitory activity and GC-MS analysis of Petrea volubilis. Int J Sci Res. 2015; 4: 190-194.
51. El-Demerdash E. Anti-inflammatory and antifibrotic effects of methyl palmitate. Toxicol Appl Pharmacol. 2011; 254(3): 238-244.
52. Pinto MEA, Araújo SG, Morais MI, Nívea PS, Lima CM, Rosa CA, et al. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. Ann Acad Bras Cienc. 2017; 89 (3): 1671-1681.
53. Kuppuswamy KM, Bhavana J, Sumathy A. GC-MS analysis of chloroform extract of Croton bonplandianum. Int J Pharm Bio Sci. 2013; 4(4): 613-617.
54. Alonso-Castro AJ, Serrano‐Vega R, Pérez Gutiérrez S, Isiordia‐Espinoza MA, Solorio‐Alvarado CR. Myristic acid reduces skin inflammation and nociception. J Food Biochem. 2022; 46(1): e14013.
55. Keskin C, Yavuz M, Kaçar S. Determination of fatty acid compositions of total lipid, phospholipid and triacylglycerol fractions of aboveground parts of four species of the genus Hyoscyamus. J Chem Res. 2016; 1(5): 1-8.
56. Gonbad RA, Afzan A, Karimi E, Sinniah UR, Swamy MK. Phytoconstituents and antioxidant properties among commercial tea (Camellia sinensis L.) clones of Iran. Electron J Biotechnol. 2015; 18(6): 433-438.
57. Banothu V, Neelagiri C, Adepally U, Lingam J, Bommareddy K. Phytochemical screening and evaluation of in vitro antioxidant and antimicrobial activities of the indigenous medicinal plant Albizia odoratissima. Pharm Biol. 2017; 55: 1155-1161.
58. Tiwary BK, Bihani S, Kumar A, Chakraborty R, Ghosh R. The in vitro cytotoxic activity of ethno-pharmacological important plants of Darjeeling district of West Bengal against different human cancer cell lines. BMC Complem Altern Med. 2015; 15-22.
59. Vijayaraghavan K, Rajkumar J, Seyed MA. Phytochemical screening, free radical scavenging and antimicrobial potential of Chromolaena odorata leaf extracts against pathogenic bacterium in wound infections- a multispectrum perspective. Biocatal Agric Biotechnol. 2018; 15: 103-112.

This work is licensed under a Creative Commons Attribution 4.0 International License.




