Analysis of early onset of Alzheimer's disease genes: disease causing and risk factors
Abstract
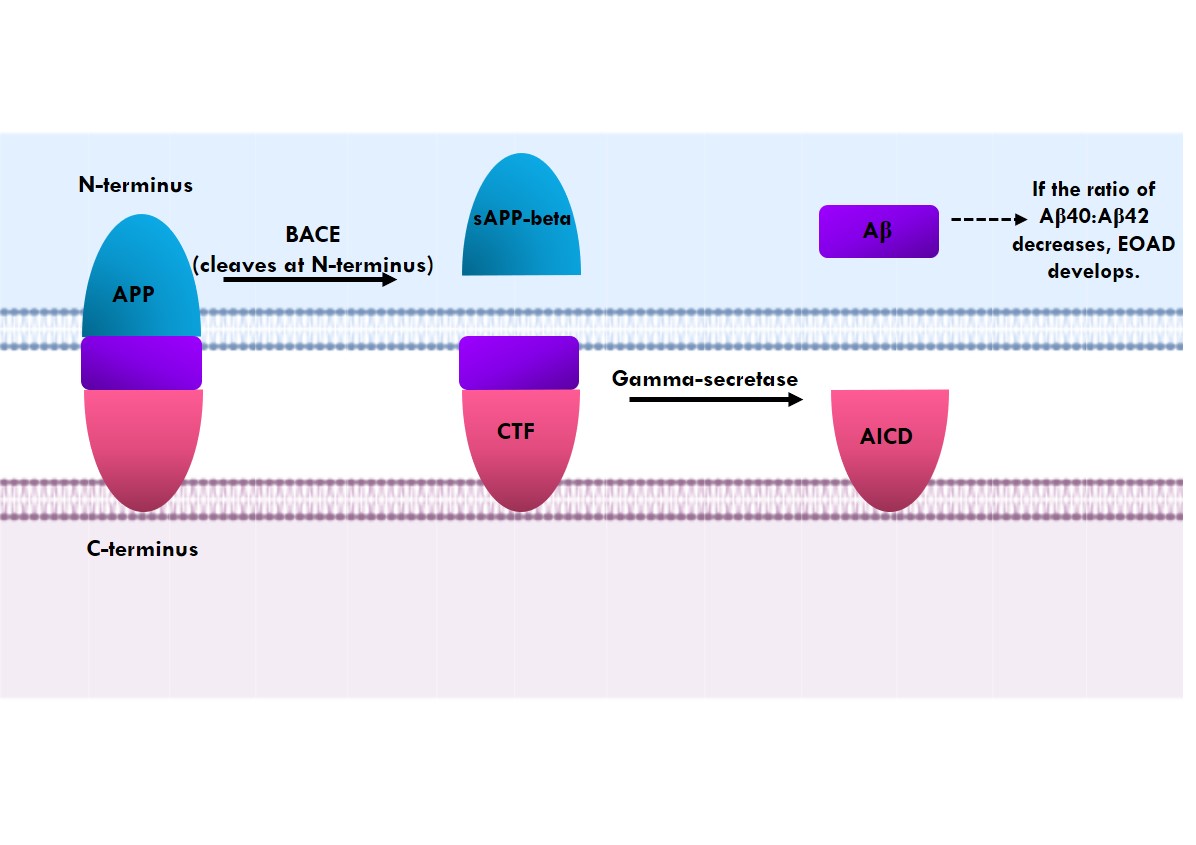
Alzheimer's disease is on the rise around the globe and is ranked sixth in the United States as the leading cause of death. It is a progressive neurodegenerative disease and the main causes of dementia. It is often characterized by symptoms such as lack of memory, agitation, restlessness, changes in personality, inability to perform everyday tasks, and impairment of speech. There are two forms of Alzheimer's disease: early onset of Alzheimer's disease occurring before 65 years of age, manifesting in 5-10% of the population, and late-onset of Alzheimer's disease manifesting after 65 years of age. In this study, the role of single nucleotide polymorphism in Alzheimer's disease using genome-wide association studies was investigated. Further, mutations underlying early onset of Alzheimer's disease were analyzed and it was found that mutations in the six genes APP, PSEN1, PSEN2, MAPT, GRN and PRNP resulted in the structural and functional protein modifications. These altered amino acids in early onset of Alzheimer's disease contribute to its pathogenesis. A single change in these genes is inherited in an autosomal dominant manner and might lead to early onset of Alzheimer's disease, however sporadic cases have also been identified.
Downloads
References
2. Tellechea P, Pujol N, Esteve-Belloch P, Echeveste B, García-Eulate MR, Arbizu J, Riverol M. Early- and late-onset Alzheimer disease: Are they the same entity? Enfermedad de Alzheimer de inicio precoz y de inicio tardío: ¿son la misma entidad? Neurologia. 2018; 33(4): 244-253.
3. Alzheimer’s Disease fact sheet, National Institute on Aging https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet
4. Dai M, Zheng H, Zeng L, Zhang Y. The genes associated with early-onset Alzheimer’s disease. Oncotarget. 2018; 9: 15132-15143.
5. Roks G, Dermaut B, Heutink P, Julliams A, Backhovens H, Van de Broeck M, et al. Mutation screening of the tau gene in patients with early-onset Alzheimer's disease. Neurosci Lett. 1999; 277(2):137-139.
6. de Paula VJR, Guimarães FM, Diniz BS, Forlenza OV. Neurobiological pathways to Alzheimer's disease: Amyloid-beta, TAU protein or both? Dement Neuropsychol. 2009; 3(3): 188-194.
7. Weller J, Budson A. Current understanding of Alzheimer's disease diagnosis and treatment. F1000Res. 2018; 7: 1161.
8. National Institute of Health, U.S Department of Health and Human Services https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet
9. Zhang Yw, Thompson R, Zhang H, Xu H. APP processing in Alzheimer's disease. Mol Brain. 2011; 4: 3.
10. Brouwers N, Sleegers K, Van Broeckhoven C. Molecular genetics of Alzheimer's disease: an update. Ann Med. 2008; 40(8): 562-83.
11. Dai M, Zheng H, Zeng L, Zhang Y. The genes associated with early-onset Alzheimer’s disease. Oncotarget. 2018; 9: 15132-15143.
12. Deng L, Haynes PA, Wu Y, Amirkhani A, Kamath KS, Wu JX, et al. Amyloid-beta peptide neurotoxicity in human neuronal cells is associated with modulation of insulin-like growth factor transport, lysosomal machinery and extracellular matrix receptor interactions. Neural Regen Res. 2020; 15(11): 2131-2142.
13. Tcw J, Goate AM. Genetics of β-Amyloid Precursor Protein in Alzheimer's Disease. Cold Spring Harb Perspect Med. 2017; 7(6): a024539.
14. Bekris LM, Yu CE, Bird TD, Tsuang DW. Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol. 2010; 23(4): 213-227.
15. Chen GF, Xu TH, Yan Y, Zhou YR, Jiang Y, Melcher K, Xu HE. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin. 2017; 38(9): 1205-1235.
16. De Strooper B. Loss-of-function presenilin mutations in Alzheimer disease. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007; 8(2): 141-146.
17. Giri M, Zhang M, Lü Y. Genes associated with Alzheimer's disease: an overview and current status. Clin Interv Aging. 2016; 11: 665-681.
18. Zhang S, Zhang M, Cai F, Song W. Biological function of Presenilin and its role in AD pathogenesis. Transl Neurodegener. 2013; 2: 15.
19. Cai Y, An SS, Kim S. Mutations in presenilin 2 and its implications in Alzheimer's disease and other dementia-associated disorders. Clin Interv Aging. 2015; 10: 1163-1172.
20. Giau VV, Bagyinszky E, Youn YC, An SSA, Kim S. APP, PSEN1, and PSEN2 Mutations in Asian Patients with Early-Onset Alzheimer Disease. Int J Mol Sci. 2019; 20(19): 4757.
21. Jun G, Ibrahim-Verbaas CA, Vronskaya M, Lambert JC, Chung J, Naj AC, et al. A novel Alzheimer disease locus located near the gene encoding tau protein. Mol Psychiatry. 2016; 21(1):108-117.
22. Alonso AD, Cohen LS, Corbo C, Morozova V, ElIdrissi A, Phillips G, Kleiman FE. Hyperphosphorylation of Tau Associates with changes in Its function beyond microtubule stability. Front Cell Neurosci. 2018; 12: 338.
23. Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010; 7(8): 656-664.
24. Kar S, Fan J, Smith MJ, Goedert M, Amos LA. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO. 2003; 22(1): 70-77.
25. Townley RA, Boeve BF, Benarroch EE. Progranulin: Functions and neurologic correlations. Neurology. 2018; 90(3): 118-125.
26. Nicholson AM, Finch NA, Almeida M, Perkerson RB, van Blitterswijk M, Wojtas A, et al. Prosaposin is a regulator of progranulin levels and oligomerization. Nat Commun. 2016; 7: 11992.
27. GRN-related frontotemporal lobar degeneration, Genetics Home Reference.
28. Mukherjee O, Wang J, Gitcho, M, Chakraverty S, Taylor-Reinwald L, Shears S, et al. Molecular characterization of novel progranulin (GRN) mutations in frontotemporal dementia. Human mutation. 2008; 29(4): 512-521.
29. Arrant AE, Roth, JR, Boyle NR, Kashyap SN, Hoffmann MQ, Murchison CF, et al. Impaired β-glucocerebrosidase activity and processing in frontotemporal dementia due to progranulin mutations. Acta Neuropathol Commun. 2019; 7: 218.
30. Bernardi L, Bruni AC. Mutations in Prion Protein Gene: Pathogenic Mechanisms in C-Terminal vs. N-Terminal Domain, a Review. Int J Mol Sci. 2019; 20(14): 3606.
31. Wulf, MA, Senatore A, Aguzzi A. The biological function of the cellular prion protein: an update. BMC Biol. 2017; 15: 34.
32. PRNP gene, Genetics Home Reference.
33. Bagyinszky E, Kang MJ, Pyun J, Giau VV, An SSA, Kim S. Early-onset Alzheimer's disease patient with prion (PRNP) p. Val180Ile mutation. Neuropsychiatr Dis Treat. 2019; 15: 2003-2013.

This work is licensed under a Creative Commons Attribution 4.0 International License.




