Laboratory diagnostic methods and reported outbreaks of anthrax in Ethiopia
Abstract
Anthrax is a zoonotic disease caused by Bacillus anthracis, a Gram-positive, non-motile, spore-forming bacterium. It is a globally distributed disease, having been reported from all continents that are populated heavily with animals and humans. The objectives were to review general laboratory diagnostic testing methods and reported outbreaks of anthrax in Ethiopia. Anthrax was second top zoonotic priority next to rabies and endemic in Ethiopia that may occur in May and June every year (Anthrax season) in several farming localities. Animal hosts acquire the disease through grazing, usually by ingestion or inhalation while there are three major routs of transmission: ingestion, inhalation and cutaneous. This review indicated that anthrax remains to be major public and animal health problem in Ethiopia. Although suspected cases of anthrax are reported from several districts, they are not well confirmed by laboratories. Prevention and control of anthrax in animals effectively reduces its impact on public health and the national economy. The control of anthrax outbreaks among domestic animals is primarily dependent on rapid identification and treatment of affected animals; enhanced surveillance for additional cases; implementation of control measures including quarantine, prophylaxis, vaccination and the proper disposal of dead animals with decontamination is critical.
Downloads
References
2. Shivachandra SB, Chnda MM, Reddy GBM, Hemadri D. Anthrax at a glance. Bengaluru Karanataka, ICAR-national institute of veterinary Epidemiology and disease Informatics (NIVEDI). 2016.
3. Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, et al. Anthrax as a biological weapon-medical and public health management. JAMA. 1999; 281: 1735-1745.
4. WHOCC. World Health Organization Collaborating Center or Remote Sensing and Geographic Information Systems for Public Health. 2005.
5. WHO. World Anthrax Data Site. 2013.
6. WHO. Integrated control of neglected zoonotic diseases in Africa: Applying the ‘One Health Concept’. Geneva: WHO Document Production Services, 2009.
7. Quinn PJ, Carter ME, Markey B, Carter GR. Grafos: Mosby International Limited, Clin Vet Microbiol. 1994: 49-53.
8. Dragon DC, Rennie RP. The ecology of anthrax spores: tough but not invincible. Canad Vet J. 1995; 36(5): 295-301.
9. Jackson PJ, Hugh-Jones ME, Adair DM, Green G, Hill KK, Kuske CR, et al. PCR analysis of tissue samples from the 1979 Sverdlovsk anthrax victims: the presence of multiple Bacillus anthracis strains in different victims. Proc Natl Acad Sci USA. 1999; 95: 1224-1229.
10. Pieracci EG, Hall AJ, Gharpure R, Haile A, Walelign, Deressa A, et al. prioritizing zoonotic diseases in Ethiopia using a one health approach. One Health. 2016; 2: 131-135.
11. Shiferaw G. Anthrax in Wabessa village in Dessie Zuria District of Ethiopia. Rev Sci Tech Int Epiz. 2004; 23(3): 952-956.
12. Spencer RC. Bacillus anthracis. J Clin Pathol. 2003; 56: 182-187.
13. Moayeri M, Leppla SH, Vrentas C, Pmerantsev AP, Liu S. Anthrax pathogenesis. Annu Rev Microbiol. 2015; 69: 185-208.
14. Baillie L, Read TD. Bacillus anthracis: a bug with attitude. Curr Opin Microbiol. 2001; 4: 78-81.
15. WHO. Anthrax in humans and animals. 4th edn. Geneva, 2008.
16. Turnbull PCB. Guidelines for the surveillance and control of anthrax in human and animals. Geneva Switzerland, WHO press.1998.
17. Driks A. The Bacillus anthracis spores. Mol Aspects Med. 2009; 30: 368-373.
18. Sweeny DA, Hicks CW, Cui X, Li Y, Eichacker PQ. Anthrax infection. Am J Resp Crit Care Med. 2011; 184: 1333-1341.
19. CDC. Anthrax. 2017.
20. Hugh-Jones M, Black Burn J. The ecology of Bacillus anthracis. Mol Aspects Med. 2009; 30: 356-367.
21. Misgie F, Atnaf A, Surafel KA. Review on anthrax and its public health and economic importance. Acad J Anim Dis. 2015; 4(3): 196-204.
22. Ibrahim KH, Brown G, Wright DH, Rotschafer JC. Bacillus anthracis: medical issues of biologic warfare. Pharmacotherapy. 1999; 19: 690-701.
23. Turn bull PCB, Hugh-Jones M. Cosivis O. World Health Organization activities on anthrax surveillance and control. J Appl Microbiol. 2005; 72: 318-320.
24. Weiner ZP, Glomski IJ. Updating perspectives on the initiation of Bacillus anthracis growth and dissemination through its host. Infect Immun. 2012; 80(5): 1626-1633.
25. Ramsay CN, Stirling A, Smith J, Hawkins G, Brooks T, Hood J, et al. An outbreak of infection with Bacillus anthracis in injecting drug users in Scotland. Euro Surveil. 2010; 15(3): pii: 19469.
26. Russel L, Pedersen M, Jensen AV, Soes LM, Hansen ABE. Two anthrax cases with soft tissue infection, severe oedema and sepsis in Danish heroin users. BMC Infect Dis. 2013; 13: 408-415.
27. Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004; 22: 247-256.
28. Boyer AE, Quinn CP, Beesley CA, Gallegos-Candela M, Marston CK, Cronin LX, Hoffmaster AR. Lethal factor toxemia and anti-protective antigen antibody activity in naturally acquired cutaneous anthrax. J Infect Dis. 2011; 204: 1321-1327.
29. Read T. The genome sequence of Bacillus anthracis Ames and comparison to closely related bacteria. Nature. 2003; 423(6935): 81-86.
30. Zwartouw HT, Smith H. Polyglutamic acid from Bacillus anthracis grown in vivo: structure and aggressin activity. Biochem J. 1955; 63: 437-442.
31. Makino SI, Uchida I, Terakado N, Sasakawa C, Yoshikawa M. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J Bacteriol.1989; 69: 722-730.
32. Ezzell, JW, Welkos SL. The capsule of Bacillus anthracis, a review. J Appl Microbiol.1999; 87(2): 250.
33. Hirsh, Dwight C, Yuan CZ. A text book of veterinary medicine. Blackwell science, Massachuetts, USA, 1999.
34. Goel AK. Anthrax: a disease of biowarfare and public health importance. WJCC. 2015; 3(1): 20-33.
35. Shadomy SV, Smith TL. Zoonosis update. Anthrax. AVMA. 2008; 233(1): 63-72.
36. Berg T, Suddes H, Morrice G, Hornitzky M. Comparison of PCR, culture and microscopy of blood smears for the diagnosis of anthrax in sheep and cattle. Lett Appl Microbiol. 2006; 43: 181-186.
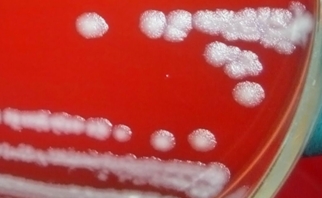
37. Mathias M, Todd Parker J. B. anthracis colony morphology. 2009.
38. Marston CK, Beesley C, Helsel L, Hoffmaster AR. Evaluation of two selective media for the isolation of Bacillus anthracis. Lett Appl Microbiol. 2008; 47: 25-30.
39. Knisely PF. Selective medium for B. anthracis. J Bacteriol. 1966; 92: 784-786.
40. Cheesbrough, M. Medical laboratory manual for tropical countries. 1st edn. Microbiology. English Language Book Society, London. 1985; 2: 400-480.
41. Todar K. Bacillus anthracis and anthrax. 2012.
42. Bell JH. On anthrax and anthracaemia in wool-sorters, heifers, and sheep. Brit Med J. 1880; 2: 656-657.
43. Eurich FW. Anthrax in the woollen industry, with special reference to bradford. Proc R Soc Med. 1913; 6: 219-240.
44. Laforce FM. Woolsorters’ disease in England. Bull N Y Acad Med. 1978; 54: 956-963.
45. Boyer AE, Quinn CP, Adrian R, Woolfitt AR, Pirkle JL, Williams LG, et al. Detection and quantification of anthrax lethal factor in serum by mass spectrometry. Anal Chem. 2007; 79(22): 8463-8470.
46. Klee SR, Nattermann H, Becker S, Urban-Schriefer M, Franz T, Jacob D, et al. Evaluation of different methods to discriminate Bacillus anthracis from other bacteria of the Bacillus cereus group. J Bacteriol. 2006; 100: 673-681.
47. Klee SR, Ozel M, Appel B, Boesch C, Ellerbrok H, Jacob D, et al. Characterization of Bacillus anthracis-like bacteria isolated from wild great apes from Cote d’Ivoire and Cameroon. J Bacteriol. 2006; 188: 5333-5344.
48. Daffonchio D, Raddadi N, Merabishvili M, Cherif A, Carmagnola L, Brusetti L, et al. Strategy for identification of Bacillus cereus and Bacillus thuringiensis strains closely related to Bacillus anthracis. Appl Env Microbiol. 2006; 72: 1295-1301.
49. Olsen J, Skogan G, Fykse E, Rawlinson E, Tomaso H, Granum P, et al. Genetic distribution of 295 Bacillus cereus group members based on adk-screening in combination with MLST (multilocus sequence typing) used for validating a primer targeting a chromosomal locus in B. anthracis. J Microbiol Method. 2007; 71: 265-274.
50. Keim P, Price L, Klevytska A, Smith K, Schupp J, Okinaka R, et al. Multiple-locus variable-number tandem repeat analysis reveals genetic relationships within Bacillus anthracis. J Bacteriol. 2000; 182: 2928-2936.
51. Lista F, Faggioni G, Valjevac S, Ciammaruconi A, Vaissaire J, le Doujet C, et al. Genotyping of Bacillus anthracis strains based on automated capillary 25-loci multiple locus variable-number tandem repeats analysis. BMC Microbiol. 2006; 6: 33.
52. Stratilo C, Lewis C, Bryden L, Mulvey M, Bader D. Single-nucleotide repeat analysis for subtyping Bacillus anthracis isolates. J Clin Microbiol. 2006; 44: 777-782.
53. Easterday W, Van Ert M, Simonson T, Wagner D, Kenefic LJ, Allender C, et al. Use of single nucleotide polymorphisms in the plcR gene for specific identification of Bacillus anthracis. J Clin Microbiol. 2005; 43:1995-1997.
54. Hoffmaster A, Meyer R, Bowen M, Marston C, Weyant R, Thurman K, et al. Evaluation and validation of a real-time polymerase chain reaction assay for rapid identification of Bacillus anthracis. Emerg Infect Dis. 2002; 8: 1178-1182.
55. Hurtle W, Bode E, Kulesh D, Kaplan R, Garrison J, Bridge D, et al. Detection of the Bacillus anthracis gyrA gene by using a minor groove binder probe. J Clin Microbiol. 2004; 42: 179-185.
56. Marston C, Gee J, Popovic T, Hoffmaster A. Molecular approaches to identify and differentiate Bacillus anthracis from phenotypically similar Bacillus species isolates. BMC Microbiol. 2006; 6: 22.
57. Qi Y, Patra G, Liang X, Williams L, Rose S, Redkar R, et al. Utilization of the rpo B gene as a specific chromosomal marker for real-time PCR detection of Bacillus anthracis. Appl Env Microbiol. 2001; 67: 3720-3727.
58. Ramisse V, Patra G, Vaissaire J, Mock M. The Ba813 chromosomal DNA sequence effectively traces the whole Bacillus anthracis community. J Appl Microbiol. 1999; 87: 224-228.
59. Hoffmaster AR, Ravel J, Rasko DA, Chapman GD, Chute MD, Marston CK, et al. Identification of anthrax toxin genes in a Bacillus cereus associated with an illness resembling inhalation anthrax. PNAS. 2004; 101: 8449-8454.
60. Pannucci J, Okinaka R, Sabin R, Kuske C. Bacillus anthracis pXO1 plasmid sequence conservation among closely related bacterial species. J Bacteriol. 2002; 184: 134-141.
61. Hugh-Jones M, de Vos V. Anthrax and wildlife. Rev Sci Tech. 2002; 21: 359-383.
62. Bahiru G, Bekele A, Seraw B, Boulanger L, Ali A. Human and animal anthrax in Ethiopia: A retrospective record review 2009-2013. Vet J. 2016; 20(2): 75-85.
63. Eshete T, Chali G, Wakshum T, Tafa S, Husen M, Deneke Y. Retrospective study on the epidemiology of bovine anthrax in Elu Aba Bor Zone, South West Ethiopia. Glob Vet. 2017; 19(4): 590-595.
64. Shiferaw F, Abdicho S, Gopilo A, Laurenson MK. Anthrax outbreak in Mago National Park, southern Ethiopia. Vet Rec. 2002; 150: 318-320.
65. Gelaw Y, Asaminew T. Periocular cutaneous anthrax in Jimma Zone, Southwest Ethiopia: a case series. BMC Res Notes. 2013; 6: 313.
66. Pérez-Tanoira R, Ramos JM, Prieto-Pérez L, Tesfamariam A. Balcha S, Tissiano G, et al. Diagnosis of cutaneous anthrax in resource-poor settings in West Arsi Province, Ethiopia. Ann Agric Environ Med. 2017; 24(4): 712-715.
67. Ethiopian Public Health Institute Center for Public Health Emergency Management. Ethiopia Wkly Epi Bull. 2018; 4(19): 1-19.